The first report about the recall of some Panado syrups by pharmaceutical company Adcock Ingram Limited was on Friday evening, 6 December 2024.
Many who initially saw reports on the recall of some grape-flavoured Panado syrups took to the internet to find out more information. However, nothing could be found anywhere online at the time.
The statement was only later published in a report by Cape Town Etc on 7 December 2024. This left many asking questions why the recall wasn’t publicised as it should and demanded more details.
ABOUT THE PANADO SYRUP RECALL
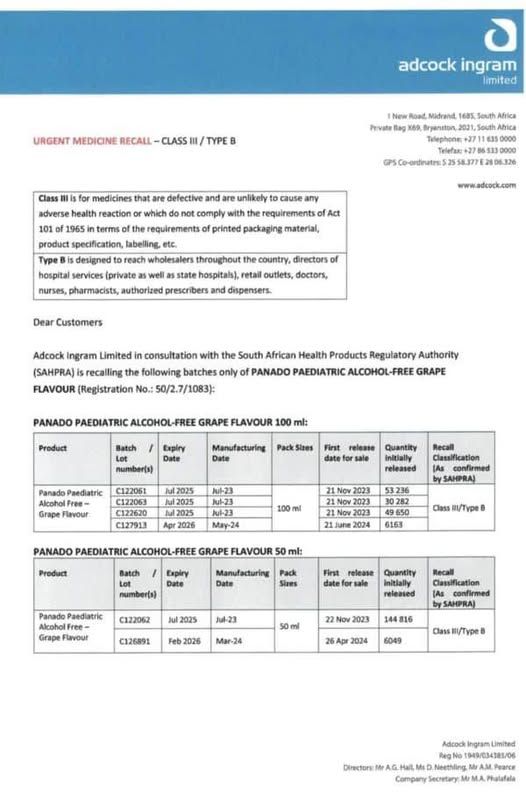
Cape Town Etc reported that Adcock Ingram Limited had confirmed that a batch of 50ml and 100ml bottles of grape-flavoured alcohol-free Panado Paediatric syrup has been recalled. The reason stated is that the products had a quality issue with its active ingredient, paracetamol. The products are said to contain precipitation in the paracetamol affecting its appearance. The expiry dates on the affected products are:
- July 2025
- February 2026
- April 2026
Adcock Ingram said there is minimal health risk to those who have taken the product as they have classified it Class III.
SCARED PARENTS FRANTICALLY SEARCHED ONLINE IF THEIR BATCH WAS AFFECTED
After not being able find any statement or information on the Panado syrup recall online, some parents came up with creative ways to know if their batch is affected. An X user who shared that she saw a report about the recall just after giving her child the product emotionally explained her dilemma.
@02Bester wrote:
“I had just given my child some grape Panado when I saw this “news article” needless to say I was panicked could not find ANY other news outlet without a paywall to find out if I had just poisoned my child. Did an image search found the batch numbers. Low and behold it’s my batch.”
Others said such as @joescot89898436 said: “That is the batch for abo my frendo,” while @Chaptervalliant wrote “Wait what? Grape flavoured? I………”
SOME LEFT WITH MORE QUESTIONS THAN ANSWERS
The only other place where the statement for the recall could be found was on the Facebook page We Are South Africans. The post left some with more questions than answers, which they shared in the comment section.
Some asked: “If the syrup didn’t meet the specifications why put on the market so our children can get SICK!!???” and “It’s not purple but See through is that the only issue?”
‘IRRESPONSIBLE NOT TO PUBLISH FULL DETAILS’
Some also stated that the company was irresponsible and asked for more details. They wrote on X:
@FionaClaytonZA – “Irresponsible not to publish full details. Information not on SAHPRA or AIL websites. @wendyknowler”
@bertje_sa – “They should give the comprehensive reason for the recall & the possible DANGER of the medication: because alot of people might have consumed it! Reason urgently needed!”
@nwaEleberi – “At least explain “precipitation”….people in the hot and dry Limpopo are anxiously awaiting precipitations….Chemistry/ Pharmacy: (active) ingredient remains separated and is not resolved(?????)”
WHAT TO DO IF YOU HAVE THE AFFECTED BATCH?
The company called on the public to refrain from using products from the batch with these expiry dates. They should also return them for a refund or exchange. According to their statement, those with questions or enquiries about the recall can contact them on 0860 ADCOCK (232625).
HAVE YOU BEEN ABLE TO FIND INFORMATION ONLINE ON THE PANADO SYRUP RECALL?
Let us know by clicking on the comment tab below this article or by emailing info@thesouthafrican.com or sending a WhatsApp to 060 011 021 1.
Subscribe to The South African website’s newsletters and follow us on @TheSAnews on X and The South African on Facebook for the latest news.